

產(chǎn)品中心


Overview
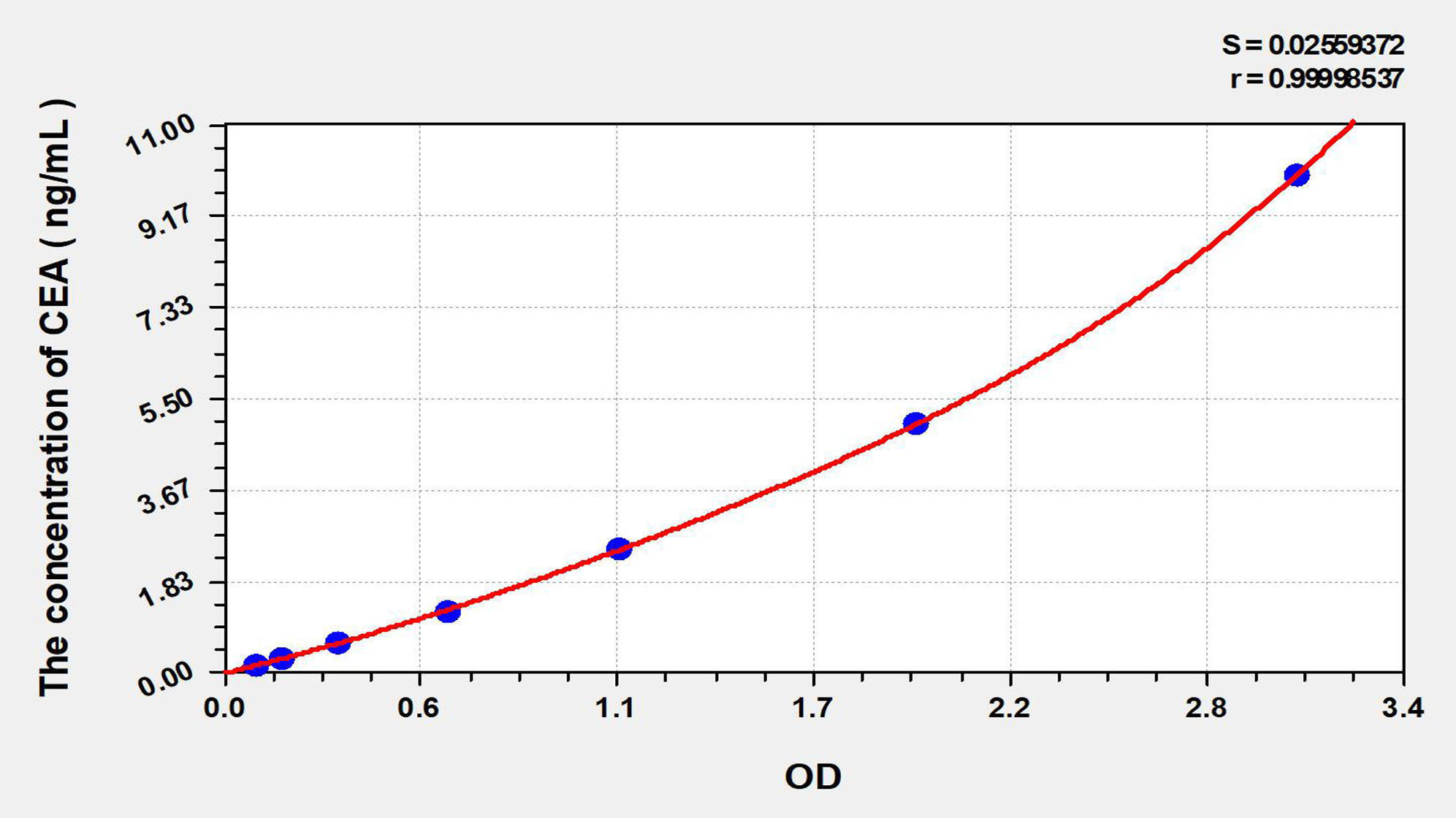
標(biāo)準(zhǔn)曲線
| Concentration (ng/mL) | OD | Corrected OD |
|---|---|---|
| 10.00 | 3.106 | 3.055 |
| 5.00 | 2.024 | 1.973 |
| 2.50 | 1.179 | 1.129 |
| 1.25 | 0.691 | 0.641 |
| 0.63 | 0.372 | 0.322 |
| 0.32 | 0.217 | 0.167 |
| 0.16 | 0.144 | 0.094 |
| 0.00 | 0.050 | 0.000 |
精密度
Intra-assay Precision (Precision within an assay):CV%<8%
Three samples of known concentration were tested twenty times on one plate to assess intra-assay precision.
Inter-assay Precision (Precision between assays):CV%<10%
Three samples of known concentration were tested in forty separate assays to assess inter-assay precision.
回收率
Matrices listed below were spiked with certain level of recombinant CEA and the recovery rates were calculated by comparing the measured value to the expected amount of CEA in samples.
| Matrix | Recovery range | Average |
|---|---|---|
| serum(n=5) | 92-110% | 101% |
| EDTA plasma(n=5) | 88-105% | 95% |
| Heparin plasma(n=5) | 90-102% | 98% |
線性
The linearity of the kit was assayed by testing samples spiked with appropriate concentration of CEA and their serial dilutions. The results were demonstrated by the percentage of calculated concentration to the expected.
| Matrix | 1:2 | 1:4 | 1:8 | 1:16 |
|---|---|---|---|---|
| serum(n=5) | 93-105% | 85-102% | 91-110% | 91-108% |
| EDTA plasma(n=5) | 90-104% | 90-106% | 88-108% | 92-109% |
| Heparin plasma(n=5) | 88-103% | 94-108% | 95-105% | 88-107% |
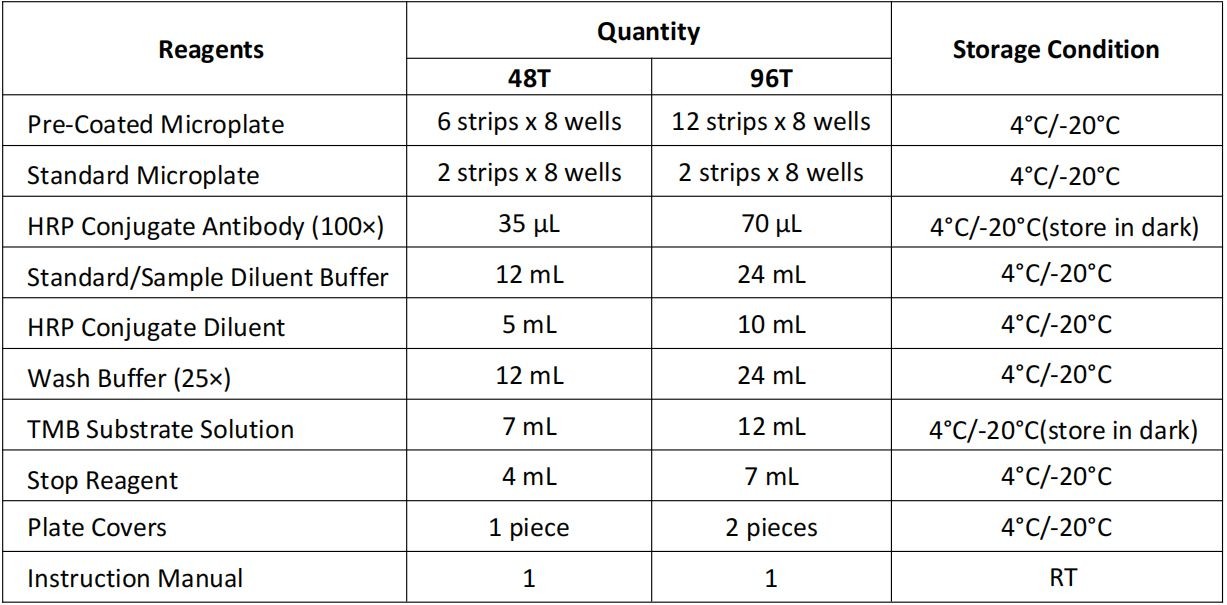
產(chǎn)品成分表
關(guān)閉
在線咨詢
Online consultation
-
在線咨詢
-
技術(shù)支持

關(guān)注微信公眾號

 下載說明 ①
下載說明 ①


